Track Categories
The track category is the heading under which your abstract will be reviewed and later published in the conference printed matters if accepted. During the submission process, you will be asked to select one track category for your abstract.
The Science of Crystals or, Crystallography represents the nature of a crystal and mostly their Atomic Structure, is very crucial for most of the practicing Scientists whose research work relates with materials and their structures. For instance, Crystallography or, Crystal Structure is being used by Chemists to discover and synthesized new chemical compounds and to change its physical properties. Crystallography is used by most of the Pharmaceuticals and drug discovering companies to make useful modifications in drugs. In addition, Crystallography helps to research into how drugs target proteins, the molecules that are essential for living organisms to function properly. Materials Scientist depends on Crystallography to study new materials having many Industrial applications. Crystals of Lithium niobate are used in the telecom markets.
Crystallography is a scientific discipline in its own right. Similarly, Crystallographers have their own international union and their own systems of Nomenclature and Notation. Crystallography can be found in all science aspects like Chemistry, Physics, Biology, Materials science and Mathematics, as well as in many industries.
- Track 1-1Small Molecule Crystallography: Novel Structures and General Interest
- Track 1-2Computational Crystallography
- Track 1-3Supermolecular Crystallography
- Track 1-4Polymer Crystallization
- Track 1-5Nano Crystallography
- Track 1-6Electron Crystallography
- Track 1-7Crystallization
Chemical Crystallography is a use of diffraction methods to the investigation of basic science. An incessant reason for existing is the recognizable proof of common items, or of the results of manufactured science tests; however point by point sub-atomic geometry, intermolecular collaborations and supreme designs can likewise be considered. Structures can be examined as an element of temperature, weight or the utilization of electromagnetic radiation, or attractive or electric field: such studies involves just little minority of the aggregate. The utilization of single precious stone X-beam diffraction to decide the structure of a concoction compound has been generally delegated 'Substance Crystallography'. The strategies, the exactness in analyses combined with the modem PC contraptions and advances in innovation makes this branch of science an unequivocal supplier of precise and exact estimations of sub-atomic measurements. Structure assurance by powder diffraction, precious stone designing, charge thickness examination and studies on atoms in energized states are the late additional items.
- Track 2-1Engineering of Crystalline and Non-crystalline Solids
- Track 2-2Structure and Properties of Functional Materials
- Track 2-3Metal-organic Frameworks and Organic: Inorganic Hybrid Materials
- Track 2-4Reactions and Dynamics in the Solid State
- Track 2-5Chemical Crystallography: General Interest
- Track 2-6Organic & Inorganic Crystals
- Track 2-7Mounting the Crystal
Precious stones are generally connected with having normally grown, level and smooth outer countenances. It has for quite some time been perceived that this confirmation of outside normality is identified with the consistency of inside structure. Diffraction strategies are presently accessible which give substantially more data about the inside structure of precious stones, and it is perceived that interior request can exist with no outside confirmation for it.
- Track 3-1Ions and salts
- Track 3-2Chemical shift interaction
- Track 3-3Functional Crystals
- Track 3-4Metal-Organic Frameworks (MOFs)
- Track 3-5Pharmaceutical Co-crystals
- Track 3-6Porous and Liquid Crystals
- Track 3-7Nano-materials and Nanotechnology
It ought to be obvious that all matter is made of iotas. From the intermittent table, it can be seen that there are just around 100 various types of molecules in the whole Universe. These same 100 molecules shape a great many distinctive substances running from the air we inhale to the metal used to bolster tall structures. Metals carry on uniquely in contrast to pottery, and earthenware production act uniquely in contrast to polymers. The properties of matter rely on upon which iotas are utilized and how they are fortified together. The structure of materials can be grouped by the general extent of different elements being considered.
The nuclear structure basically influences the substance, physical, warm, electrical, attractive, and optical properties. The microstructure and macrostructure can likewise influence these properties yet they for the most part largely affect mechanical properties and on the rate of concoction response. The properties of a material offer intimations with regards to the structure of the material. The quality of metals proposes that these molecules are held together by solid bonds. In any case, these bonds should likewise permit molecules to move since metals are additionally typically formable. To comprehend the structure of a material, the sort of particles present, and how the iotas are organized and fortified must be known. We should first take a gander at nuclear holding.
- Track 4-1Early organic and small biological molecules
- Track 4-2Mineralogy and metallurgy
- Track 4-3Metals and Alloys
- Track 4-4Ceramics and Polymers
- Track 4-5Thin films
- Track 4-6Quasi-crystals
- Track 4-7Amorphous Materials
- Track 4-8Bulk Nitride Crystals
- Track 4-9Novel crystallization strategies for XFEL studies
X-ray crystallography is a method used to determine the properties of a crystal which includes atomic and molecular structure, in which the crystalline atoms cause a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information.
Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various materials, especially minerals and alloys. The method also revealed the structure and function of many biological molecules, including vitamins, drugs, proteins and nucleic acids such as DNA. X-ray crystallography is still the chief method for characterizing the atomic structure of new materials and in discerning materials that appear similar by other experiments. X-ray crystal structures can also account for unusual electronic or elastic properties of a material, shed light on chemical interactions and processes, or serve as the basis for designing pharmaceuticals against diseases.
- Track 5-1X-ray diffraction
- Track 5-2Single-Crystal X-ray diffraction
- Track 5-3Fourier transformation
- Track 5-4Fiber diffraction
- Track 5-5Powder diffraction
- Track 5-6Contributions to Chemistry and Material Science
- Track 5-7Elastic vs. Inelastic Scattering
- Track 5-8Applications of X-ray diffraction
X-beams are utilized to examine the basic properties of solids, fluids or gels. Photons interface with electrons, and give data about the vacillations of electronic densities in the matter. A run of the mill test set-up is appeared on Figure 1: a monochromatic light emission wave vector ki is chosen and falls on the specimen. The scattered power is gathered as a component of the alleged dissipating point 2θ. Versatile cooperation’s are described by zero vitality exchanges, with the end goal that the last wave vector kf is equivalent in modulus to ki. The applicable parameter to examine the collaboration is the force exchange or diffusing vector q=ki-kf, characterized by:
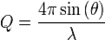
The scattered force I(q) is the Fourier Transform of g(r), the connection capacity of the electronic thickness r(r), which compares to the likelihood to discover a scatterer at position r in the specimen if another scatterer is situated at position 0 : flexible x-beam dissipating tests uncover the spatial relationships in the example. Little edge diffusing analyses are intended to quantify I(q) at little scrambling vectors q»(4p/l)q, with 2q going from couple of small scale radians to a ten of radians, to examine frameworks with trademark sizes running from crystallographic separations (few Å) to colloidal sizes (up to couple of microns).
- Track 6-1Recent Developments in Crystal Growth
- Track 6-2Crystal growth kinetics and mechanisms
- Track 6-3Crystal morphology
- Track 6-4Diamonds growth
- Track 6-5Organic Crystal Scintillators
- Track 6-6Phase Transitions: seeding, growth, transport
- Track 6-7Melt Growth 1: hydrodynamic concepts, external fields
- Track 6-8Melt Growth 2: microgravity and modeling
- Track 6-9Aqueous solution, ammonothermal growth
- Track 6-10Growth from melt solution, liquid phase epitaxy
Nuclear magnetic resonance crystallography (NMR crystallography) is a method which utilizes primarily NMR spectroscopy to determine the structure of solid materials on the atomic scale. Thus, solid-state NMR spectroscopy would be used primarily, possibly supplemented by quantum chemistry calculations (e.g. density functional theory), powder diffraction etc. If suitable crystals can be grown, any crystallographic method would generally be preferred to determine the crystal structure comprising in case of organic compounds the molecular structures and molecular packing. The main interest in NMR crystallography is in microcrystalline materials which are amenable to this method but not to X-ray, neutron and electron diffraction. This is largely because interactions of comparably short range are measured in NMR crystallography.
- Track 7-1Dipolar interaction
- Track 7-2Non-covalent interactions
- Track 7-3Solid-State NMR
- Track 7-4Crystal Structure Refinements
- Track 7-5Applications of NMR
- Track 7-6NMR spectroscopy
- Track 7-7NMR crystallography
Spectroscopy is the study of the interaction between matter and electromagnetic radiation. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, by a prism. Later the concept was expanded greatly to include any interaction with radiative energy as a function of its wavelength or frequency. Spectroscopic data are often represented by an emission spectrum, a plot of the response of interest as a function of wavelength or frequency.
One of the central concepts in spectroscopy is a resonance and its corresponding resonant frequency. Resonances were first characterized in mechanical systems such as pendulums. Mechanical systems that vibrate or oscillate will experience large amplitude oscillations when they are driven at their resonant frequency. A plot of amplitude vs. excitation frequency will have a peak centered at the resonance frequency. This plot is one type of spectrum, with the peak often referred to as a spectral line, and most spectral lines have a similar appearance.
Spectra of atoms and molecules often consist of a series of spectral lines, each one representing a resonance between two different quantum states. The explanation of these series, and the spectral patterns associated with them, were one of the experimental enigmas that drove the development and acceptance of quantum mechanics. The hydrogen spectral series in particular was first successfully explained by the Rutherford-Bohr quantum model of the hydrogen atom. In some cases spectral lines are well separated and distinguishable, but spectral lines can also overlap and appear to be a single transition if the density of energy states is high enough. Named series of lines include the principal, sharp, diffuse and fundamental series.
- Track 8-1Symmetry and Molecular Spectroscopy
- Track 8-2Spectroscopy and Molecular Structure
- Track 8-3Infrared Spectroscopy Life
- Track 8-4X-ray photoelectron spectroscopy (XPS)
- Track 8-5Small Molecule Spectroscopy and Dynamics
- Track 8-6Photoemission spectroscopy
- Track 8-7Raman spectroscopy
- Track 8-8Time-Stretch Spectroscopy
- Track 8-9Ultraviolet photoelectron spectroscopy (UPS)
- Track 8-10Ultraviolet visible spectroscopy
- Track 8-11Vibrational circular dichroism Spectroscopy
- Track 8-12Dynamic Force Spectroscopy
Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material. A sample to be examined is placed in a beam of thermal or cold neutrons to obtain a diffraction pattern that provides information of the structure of the material. The technique is similar to X-ray diffraction but due to their different scattering properties, neutrons and X-rays provide complementary information: X-Rays are suited for superficial analysis, strong x-rays from synchrotron radiation are suited for shallow depths or thin specimens, while neutrons having high penetration depth are suited for bulk samples.
- Track 9-1Nuclear scattering
- Track 9-2Magnetic scattering
- Track 9-3Hydrogen, null-scattering and contrast variation
- Track 9-4Specific Applications of Neutron Scattering
- Track 9-5Thomson scattering
Structural biology is a branch of molecular biology, biochemistry, and biophysics concerned with the molecular structure of biological macromolecules, especially amino and nucleic acids, how they acquire the structures they have, and how alterations in their structures affect their function. This subject is of great interest to biologists because macromolecules carry out most of the functions of cells, and only by coiling into specific three-dimensional shapes that they are able to perform these functions. This architecture, the "tertiary structure" of molecules, depends in a complicated way on the molecules' basic composition, or "primary structures."
Hemoglobin, the oxygen transporting protein found in red blood cells.
Biomolecules are too small to see in detail even with the most advanced light microscopes. The methods that structural biologists use to determine their structures generally involve measurements on vast numbers of identical molecules at the same time.
- Track 10-1Mass spectrometry
- Track 10-2NMR Spectroscopy
- Track 10-3Bio-Macromolecular Crystallography
- Track 10-4Bio-Macromolecular Crystallography
- Track 10-5Proteolysis
- Track 10-6Electron paramagnetic resonance (EPR)
- Track 10-7Cryo-electron microscopy (cryo-EM)
- Track 10-8Multi-angle light scattering
- Track 10-9Small angle scattering
- Track 10-10Ultrafast laser spectroscopy
- Track 10-11Structure of interfaces
Although they utilize different approaches, nuclear magnetic resonance (NMR) spectroscopy and x-ray crystallography comprise the two best means of analyzing protein structure and function at or near atomic resolution. The degree to which these techniques differ and complement each has been a source of long-standing debate. Do proteins amenable to structural analysis by NMR also crystallize well? Does crystallography provide better structural resolution? Is NMR protein analysis closer to the native state? Despite some similarities and differences, each technique excels where the other falls short, making protein NMR and x-ray crystallography two very complementary spectroscopic methods for high resolution analysis of protein structure and function.
Below is a brief summary of the basic technical aspects of protein NMR spectroscopy as compared to x-ray crystallography. A more thorough analysis for each technique can be found in existing UC Davis Wiki pages for Nuclear Magnetic Resonance (NMR) Spectroscopy and X-ray Crystallography.
In the end, protein x-ray crystallography and NMR spectroscopy are not mutually exclusive techniques; one can easily pick up where the other falls short. In analyzing NMR dynamics experiments, for example, one can greatly benefit from existing crystal structure data onto which the NMR structural data can be superimposed. Similarly, NMR structure data can be used to supplement a crystal structure with more information on the protein's dynamics, binding information, and conformational changes in solution. Because a protein that can be analyzed by NMR is not necessarily amenable to crystallization (and vice-versa), the two techniques, either alone or in conjunction with one another, serve as two of the top complementary methods for protein structure determination.
- Track 11-1Protein NMR Spectroscopy
- Track 11-2X-ray Protein Crystallography
- Track 11-3Data Analysis
- Track 11-4Protein Production
- Track 11-5Protein Stability and Crystallization
- Track 11-6Protein Size
- Track 11-7NMR Dynamics
- Track 11-8Crystallography Dynamic Structure Analysis
Crystal engineering is the design and synthesis of molecular solid state structures with desired properties, based on an understanding and use of intermolecular interactions. The two main strategies currently in use for crystal engineering are based on hydrogen bonding and coordination bonding. These may be understood with key concepts such as the supramolecular synthon and the secondary building unit.
The term ‘crystal engineering’ was first used in 1971 by Gerhard Schmidt in connection with photodimerization reactions in crystalline cinnamic acids. Since this initial use, the meaning of the term has broadened considerably to include many aspects of solid state supramolecular chemistry. A useful modern definition is that provided by Gautam Desiraju, who in 1988 defined crystal engineering as "the understanding of intermolecular interactions in the context of crystal packing and the utilization of such understanding in the design of new solids with desired physical and chemical properties." Since many of the bulk properties of molecular materials are dictated by the manner in which the molecules are ordered in the solid state, it is clear that an ability to control this ordering would afford control over these properties.
- Track 12-1Non-covalent control of structure
- Track 12-2Design of multi-component crystals
- Track 12-32D Structures
- Track 12-4Polymorphism
- Track 12-5Crystal structure prediction
- Track 12-6Property design
- Track 12-7Crystal Nets (periodic graphs)
- Track 12-8Crystal Growth & Design
Crystallography method has been a broadly utilized device for illustration of mixes present in drain and different sorts of data acquired through structure work relationship. Albeit more point by point data from X-beam investigation has been secured from substances which are normally known to be crystalline, it has been amazing to discover substances generally considered as being non-crystalline as really having a halfway crystalline structure and that this structure can be changed by warmth treatment, weight, extending, and so forth. Casein is a case of the last class of proteins. Stewart has demonstrated that even arrangements have a tendency to accept a methodical game plan of gatherings inside the arrangement. Consequently, fluid drain ought to, and shows some sort of course of action. The mineral constituent and lactose are the main genuine crystalline constituents in dairy items that can be investigated by X-beam; in any case, intriguing basic changes have been seen in butterfat, drain powder, casein and cheddar.
- Track 13-1High-Resolution Charge Density Studies
- Track 13-2Semiconductors and Insulators
- Track 13-3X-ray method for investigation of drugs
- Track 13-4X-ray method for investigation of textile fibers and polymers
- Track 13-5X-ray method for investigation of bones
- Track 13-6Spectroscopy at Fusion Reactors
- Track 13-7Pre-clinical imaging
- Track 13-8Surface Stress Measurements
- Track 13-9Photo-Crystallography
Cure monitoring of composites using optical fibers.
Estimate weathered wood exposure times using near infrared spectroscopy.
Measurement of different compounds in food samples by absorption spectroscopy both in visible and infrared spectrum.
Measurement of toxic compounds in blood samples.
Photoacoustic spectroscopy measures the sound waves produced upon the absorption of radiation.
Photothermal spectroscopy measures heat evolved upon absorption of radiation.
Pump-probe spectroscopy can use ultrafast laser pulses to measure reaction intermediates in the femtosecond timescale.
Raman optical activity spectroscopy exploits Raman scattering and optical activity effects to reveal detailed information on chiral centers in molecules.
Spin noise spectroscopy traces spontaneous fluctuations of electronic and nuclear spins.
Time-resolved spectroscopy measures the decay rate(s) of excited states using various spectroscopic methods.
Thermal infrared spectroscopy measures thermal radiation emitted from materials and surfaces and is used to determine the type of bonds present in a sample as well as their lattice environment. The techniques are widely used by organic chemists, mineralogists, and planetary scientists.
Transient grating spectroscopy measures quasiparticle propagation. It can track changes in metallic materials as they are irradiated.
- Track 14-1Spectroscopy in Environmental Analysis
- Track 14-2Spectroscopy in Biomedical Sciences
- Track 14-3Spectroscopy in Astronomy
- Track 14-4Spectroscopy in Materials Science
- Track 14-5Spectroscopy in Laser-induced Fluorescence
- Track 14-6Atomic Emission Spectroscopy (AES)
- Track 14-7Atomic Absorption Spectroscopy (AAS)
- Track 14-8Applications in Mass Spectrometry
- Track 14-9Spectroscopy in the Photon Migration Regime
Modern Analytical Chemistry is used in the analysis of light energy emitted by electrons, atoms, ions, or molecules at their ground state. Modern Analytical Chemistry deals with the determination of component structure IR spectrum is used to identify the bonds when organic compound is exposed to electro-magnetic radiation.
Modern Chemistry Formulae’s
Modern Chemistry Formulae’s for an ionic substance symbolizes one system device - the easiest rate of the compound's beneficial ions (cations) and its adverse ions (anions).Modern Chemistry Formulaes is the study and use of natural analytical structural chemistry to purify various compounds. Kinetics formula can be classified as research work ,work power, work cement chemist which is contributed to develop the modern chemistry.
Modern Experimental Chemistry
Modern Experimental Chemistry fully deals with the fundamentals of kinetics and heterogeneous catalysis in modern chemistry. Modern Experimental Chemistry are used in couple cluster method for ground and existed states, geminal wave functions embedding methods for exploring potential energy.
Modern Heterocyclic Chemistry
Modern Heterocyclic Chemistry or a heterocyclic substance with a band framework is a cyclic substance that has atoms of at least two different components as associates of its rings. Modern Heterocyclic Chemistry mainly deals with the study of heterocyclic compounds it is used in the development and increasing the relevant biological targets (enzymes, modulators).
Modern Inorganic Chemistry
Modern Inorganic Chemistry deals with the study of the features and actions of inorganic and organometallic substances. Modern Inorganic Chemistry includes all substance products except the variety natural substances (carbon based substances, usually containing C-H bonds), which are the topics of natural substance make up. Modern Inorganic Chemistry has programs in every part of the substance industry–including catalysis, materials technology, pigmentation, surfactants, coverings, medication, energy, and farming.
Modern Nuclear Chemistry
Modern Nuclear Chemistry is the study of physics and chemistry of heaviest elements their nuclear properties such as structure, reaction radioactive decay. Modern Nuclear Chemistry deals with atomic process such as ionization, x-ray emission, nuclear nomenclature, survey of nuclear decay types, nuclear chemistry.
Modern Organometallic Chemistry
Modern Organometallic Chemistry is the study of substance products containing at least one connection between an atom as well as atom of a natural substance and a steel. Organometallic substance make up brings together factors of inorganic substance make up and natural substance make up. Organometallic substances are commonly used in homogeneous catalysis. The term "metalorganics" usually represents metal-containing substances missing direct metal-carbon ties but which contain natural ligands. Metal beta-diketonates, alkoxides, and dialkylamides are associate members of this modern organometallic chemistry class.
Modern Physical Organic Chemistry
Modern Physical Organic Chemistry is mainly focused on the chemical structures and their reactivity to study their organic molecules which include the study of their rates and reactions. Modern Physical Organic Chemistry has wide applications in chemical biology, bioorganic chemistry , electro-photochemistry , polymer, supramolecular chemistry, nanotechnology and drug discovery.
Modern Stoichiochemistry is established on the law of preservation of huge where the complete huge of the reactants is equal to the complete huge of the items resulting in the understanding that the interaction among quantities of reactants and items generally type a rate of beneficial integers. Modern Stoichiochemistry studies the kinetics and Stoichiochemistry of the transition from the primary to secondary peroxidase. A hypothesis formulated that the Stoichiochemistry of regularity gene was influential in modulating the levels of expression of the targeted genus.
Modern Theoretical Chemistry
Modern Theoretical Chemistry looks for to offer details to substance and physical findings. Modern Theoretical Chemistry make up has the essential rules of science such as Coulomb's law, Kinetic power, Potential power, the virial theorem, Planck's Law, Pauli exemption concept and many others to describe but also estimate substance noticed phenomena. In order to describe a statement one has to choose the "right level of theory".
- Track 15-1Innovations in Mass Spectrometry techniques
- Track 15-2Mass Spectrometry Imaging approaches and applications
- Track 15-3Ion Mobility Spectrometry
- Track 15-4Current Brain Research with NMR Spectroscopy
- Track 15-5Advances in Chromatography and Crystallography
- Track 15-6Crystal Lattices
- Track 15-7Advanced Trends in Organic Chemistry
- Track 15-8Modern Organic Chemistry and Applications
- Track 15-9Structural Effects in Organic Electrochemistry
- Track 15-10Structural Biochemistry and Crystallography
- Track 15-11Coordination & Crystallographic Defects
- Track 15-12Macromolecular structure and function
- Track 15-13Polymer Chemistry
- Track 15-14Material Chemistry for Electrochemical capacitors
- Track 15-15Materials Synthesis